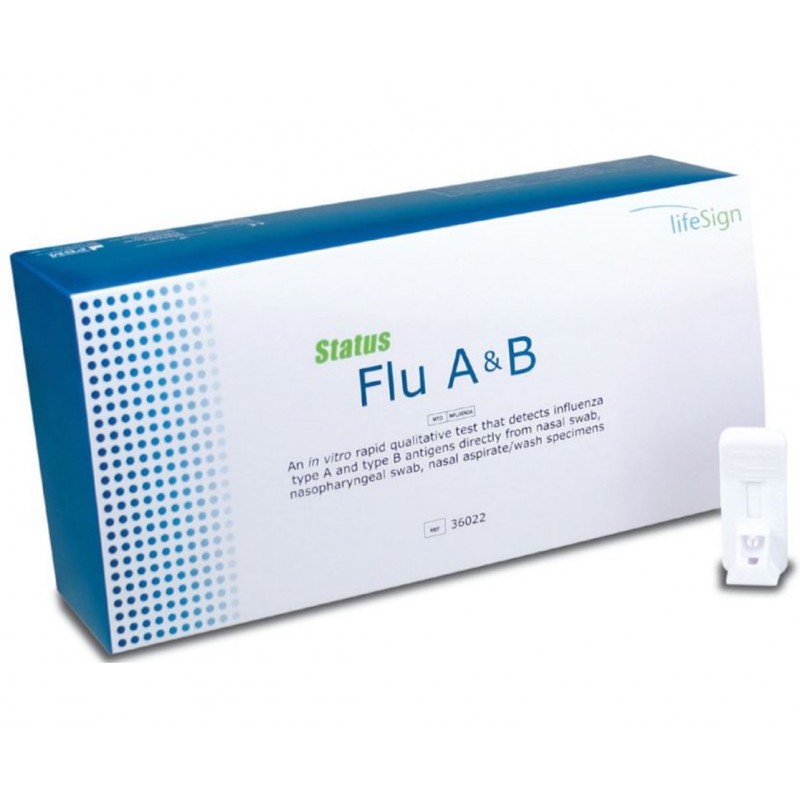
BioSign Rapid Flu A+B Test – Box of 25 Tests
$229.25
- Sold in: Box of 22 Tests
- CLIA Waived & FDA Approved
- Results in 10 Minutes
- An easy nasal swab procedure
161 in stock
BioSign Rapid Flu A+B Test
- Sold in: Box of 25 Tests
- CLIA Waived & FDA Approved
Reimbursement Information
| CPT Codes | |
| 87804 QW | Influenza A Detection |
| 87804-59 | Influenza B Detection |
An In vitro rapid qualitative test that detects influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab and nasal aspirate/wash specimens.
Features:
- Differentiates between Influenza Type A and B, on a single test device
- Produces results in 10 to 15 minutes
- Each test device includes built-in controls
- Each kit comes with external controls
- Meets FDA performance criteria for influenza tests stated in code 21 CFR 866.3328
- CLIA Complexity: Moderate complexity when used with nasal wash/aspirate samples
- CLIA Waived when used with nasal and nasopharyngeal swabs

SKU: BSP510-25
| Weight | 1.5 lbs |
|---|---|
| Dimensions | 7 × 4 × 4 in |
| Brand | |
| Product Type | Influenza A&B Test |
Be the first to review “BioSign Rapid Flu A+B Test – Box of 25 Tests” Cancel reply
Related products
Sale!









Reviews
There are no reviews yet.