Click here to download the Healthcare Provider Fact Sheet.
CorDx COVID-19 Home Test – 150 Packs, 300 Tests
$760.00
- EUA authorized for over-the-counter use
- 98.1% accuracy
- Rapid results within 10 minutes
52 in stock
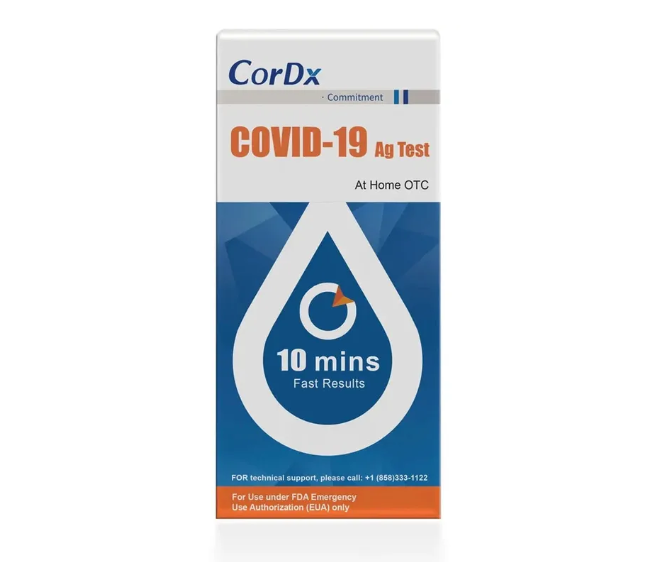
CorDx COVID-19 Home Test
- 150 Packs of 2 tests, 300 Total Tests/Case
- Expiration of Current inventory: Aug 2024
The CorDx COVID-19 Ag Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus, delivering results in just 10 minutes.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult collected anterior nasal (nares) swab samples from individuals aged two years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
Click here to access our instructions for use (IFU) healthcare provider
Click here to access our interactive quick reference instructions (QRI)
Haga clic aquí para acceder a nuestras instrucciones interactivas de referencia rápida (QRI)
FDA/EUA Advisory:
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA.
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
SKU: CORDEX-300
| Weight | 28 lbs |
|---|---|
| Dimensions | 20 × 14 × 14 in |













Reviews
There are no reviews yet.