BD Veritor SARS-CoV-2 Rapid Detection Kit – 30 Tests/box
$765.40 Original price was: $765.40.$711.25Current price is: $711.25.
- SARS-CoV-2 Rapid test
- For use with BD Veritor System
- This product is only to be used under an EUA
- Test is authorized for use at POC operating under a CLIA Certificate of Waiver, Certificate of Compliance or Certificate of Accreditation
Out of stock
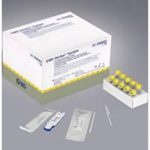
BD Veritor SARS-CoV-2 Rapid Detection Kit
- 30 Test/Box
- SKU: 256082
- ANALYZER NOT INCLUDED
The BD Veritor SARS-CoV-2 Rapid Detection Kit is a chromatographic immunoassay for the direct and qualitative detection of Coronavirus antigens in nasal swabs from patients showing signs and symptoms of COVID-19. Portable and easy to use with real-time results, it provides frontline health care workers and patients access to a quick COVID-19 diagnostic test at convenient locations like doctors’ offices, urgent care centers, and even retail pharmacies.
Easy to use with fast results: The BD Veritor SARS-CoV-2 Rapid Detection Kit comes with specimen sampling swabs, extraction reagents, and test devices. Gathered nasal swab samples are mixed with the reagent, which is then placed into the test device. After running the test for 15 minutes, the test device is then inserted into the Veritor Analyzer (purchased separately) which analyzes and displays the results. The simplified testing process boosts medical facilities’ capacity to conduct more in-patient rapid COVID-19 tests, resulting in faster identification of infectious individuals and contact tracing.
Advanced particle technology: When specimens are added to the test device, SARS-CoV-2 antigens present in the specimen bind to antibodies conjugated to detector particles in the test strip. The antigen-conjugate complexes are then captured by a line of antibodies bound to the membrane of the reaction area. The Analyzer detects a positive result when the antigen-conjugate is deposited at the Test “T” position and the Control “C” position on the test device. The instrument also analyzes and corrects for non-specific binding and detects positives that are unrecognizable by the naked eye, drastically improving specificity and reducing false-positive results.
By making COVID-19 antigen tests more accessible, the BD Veritor SARS-CoV-2 Rapid Detection Kit enables frontline healthcare workers to make better-informed decisions while the patient is still on-site, potentially slowing down the spread of the virus in the larger community.
FDA Disclaimers:
• This test is not FDA cleared or approved.
• It has been authorized by the FDA under an Emergency Use Authorization for use by authorized laboratories.
• It has been authorized only for the detection of proteins from SARS-CoV-2 and not for any other viruses or pathogens.
• This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
| Weight | 3 lbs |
|---|---|
| Dimensions | 10 × 8 × 6 in |
| Brand | |
| Product Type | COVID-19 Test |
Related products
Point of Care Tests